Magnesium. What’s in it for ATSI?
As we improve our preventative health screening practices in Remote Indigenous Health we inevitably encounter incidental abnormalities in routine pathology. Given our limited resources and developing recall systems, what do we do about them?
Hypomagnesemia is a common finding in population screening in the Torres Strait. Epidemiological studies have shown that people consuming Western-type diets are low in magnesium content, i.e. <30%–50% of the recommended daily amount.
Green vegetables are the major source of magnesium. Nuts, seeds and unprocessed cereals are also rich in magnesium. Legumes, fruit, fish and meat have an intermediate magnesium concentration. Although the rates of magnesium deficiency in Torres Strait Islanders are unknown, studies of other indigenous populations confirm low to critical rates of dietary magnesium intake.
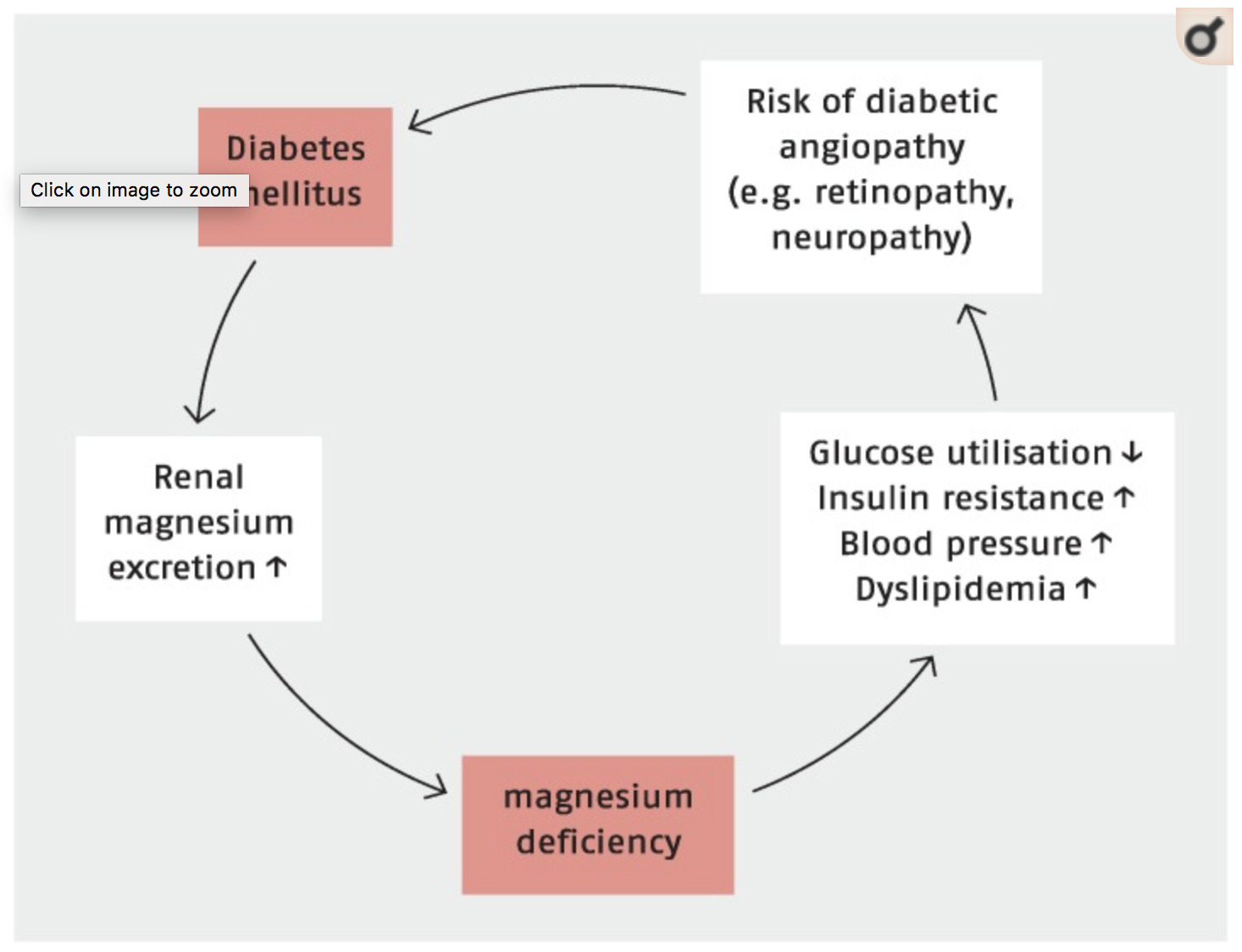
So what’s the big deal? It turns out magnesium deficiency is associated with many of the chronic diseases endemic to our indigenous population. Serum magnesium <0.75 mmol/L has been associated with atherosclerosis, hypertension, cardiac arrhythmias, stroke, alterations in lipid metabolism, insulin resistance, metabolic syndrome, type 2 diabetes mellitus and CKD. Association is all very well but is replacing a magnesium of 0.69mmol/L going to improve outcomes for my patient?
In their 2015 review article Grober et al summarise the most powerful and relevant evidence for our population; magnesium intake, dietary or supplementation, is inversely associated with the incidence of type 2 diabetes. Furthermore a higher magnesium intake may significantly lower the risk of progressing from prediabetes to manifest diabetes and there is even evidence that magnesium supplementation improves insulin sensitivity in normomagnesemic, overweight, non-diabetic subjects.
In a double-blind placebo-controlled randomized trial a total of 116 men and non-pregnant women, aged 30–65 years with hypomagnesaemia and newly diagnosed with prediabetes, were enrolled to receive either 30 mL of MgCl2 5% solution (equivalent to 382 mg of magnesium) or an inert placebo solution once daily for four months. The primary trial endpoint was the efficacy of magnesium supplementation in reducing plasma glucose levels. At the end of follow-up, fasting (86.9 ± 7.9 and 98.3 ± 4.6 mg/dL, respectively; p = 0.004) and post-load glucose (124.7 ± 33.4 and 136.7 ± 23.9 mg/dL, respectively; p = 0.03) levels, HOMA-IR indices (2.85 ± 1.0 and 4.1 ± 2.7, respectively; p = 0.04) and triglycerides (166.4 ± 90.6 and 227.0 ± 89.7, respectively; p = 0.009) were significantly decreased, whereas high density lipoprotein cholesterol (HDL) (45.6 ± 10.9 and 46.8 ± 9.2 mg/dL, respectively; p = 0.04) and serum magnesium (1.96 ± 0.27 and 1.60 ± 0.26 mg/dL, respectively; p = 0.005) levels were significantly increased in those taking MgCl2 compared with the controls. A total of 34 (29.4%) people improved their glucose status (50.8% and 7.0% in the magnesium and placebo groups, respectively; p < 0.0005)
If magnesium supplementation affects insulin sensitivity in patients with diabetes mellitus, it may also improve insulin sensitivity in obese individuals who are at risk of type 2 diabetes mellitus. Therefore, effects of magnesium supplementation in overweight, normomagnesemic individuals who had insulin resistance, but not type 2 diabetes mellitus were examined. Individuals were randomly assigned to receive either magnesium aspartate hydrochloride supplementation (n = 27) or a placebo (n = 25) for 6 months. As trial endpoints, several indices of insulin sensitivity (e.g., plasma glucose, serum insulin) were determined. Magnesium supplementation resulted in a significant improvement of fasting blood glucose and some insulin sensitivity indices compared to placebo. The results provide evidence that magnesium supplementation improves insulin sensitivity even in normomagnesemic, overweight, non-diabetic subjects emphasizing the need for an early optimization of magnesium status to prevent insulin resistance and subsequently type 2 diabetes.
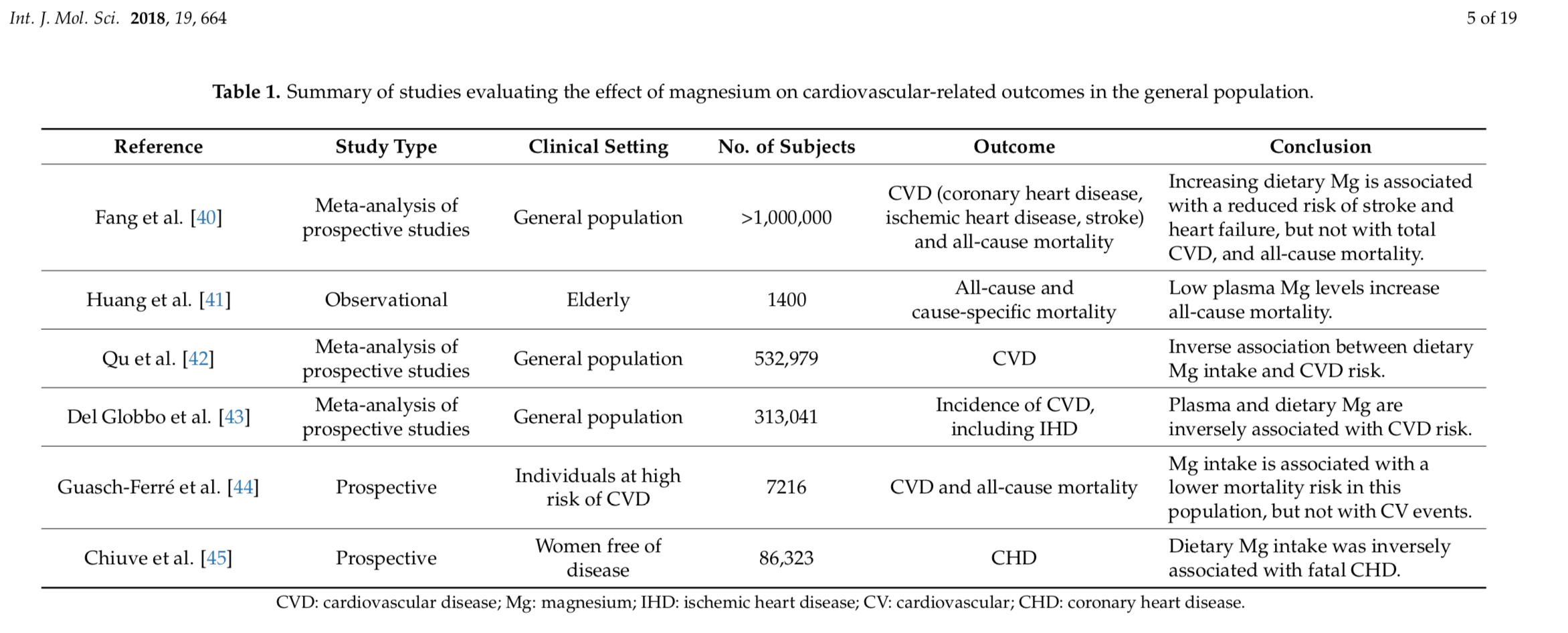
If that was not enough for us to sit up and take notice, in their 2018 review Muñoz-Castañeda etal describe improved cardiovascular outcomes and prevention of CKD progression for those on magnesium supplementation.
This review article describes how low magnesium levels have been associated with a high risk of incident CKD or end-stage renal disease. When CKD is established, hypomagnesemia predicts loss of kidney function and in diabetic nephropathy low plasma magnesium has been associated with a faster decline with progression to end-stage renal disease. Taken together, experimental evidence strongly suggests a beneficial effect of the restoration of Mg levels when it comes to cardiovascular health. However, the vast majority of observations have been originated in experimental or observational studies. Therefore, there is an unmet need for prospective clinical trials that help elucidate the impact of Mg supplements on the cardiovascular health of CKD patients.
Type 2 diabetes, CVD and CKD are three of the major contributors to the health gap in indigenous health. Armed with the above evidence we should have little hesitation in considering magnesium supplementation at every opportunity and replacing deficiency where practical. How this translates in practice in a population where adherence and pill burden already confounds therapeutic interventions is a topic for another post and another island doc, watch this space.
Further reading
http://care.diabetesjournals.org/content/26/4/1147
http://care.diabetesjournals.org/content/34/9/2116